Why Urine is an ideal feedstock to make Hydrogen
In 2012 three Nigerian girls produced a science project using Urine as a feedstock to make Hydrogen to power an electrical generator. See => https://www.nbcnews.com/tech/tech-news/african-girls-pee-powered-genera… and many scoffed saying it used more power than it produced. BUT . . . that is not what is important and nor was it true . . . see https://phys.org/news/2009-07-hydrogen-urine.html and https://phys.org/news/2017-09-army-scientists-power-urine.html because the question is reducing external energy input to gain Hydrogen and in an ideal scenario - what would be wasted energy anyway - is then used most efficiently
Per day, the average human excretes about 1.5 - 2 liters of urine consisting of 95% of water. The rest (in grams per day consists of)
# 25 g urea
# 1 g uric acid (metabolic end product of purines from DNA)
# 1.5 g creatinine (derived from creatine phosphate, the muscle booster)
# 10 g of salts, mainly NaCl
# 3 g of phosphates, citric acid, oxalic acid,
# milligrams of proteins, and urochromen, which is the compound giving rise to the yellow colour of urine.
Now, looking at this information, what makes it useful as a feedstock to make Hydrogen is the salt and urea. The salt for its conductivity as an electrolyte in water and urea for the hydrogen bonds it has which are easily freed under electrolysis in a specially built electrolyzer.
It cannot be used in a PEM design electrolyzer however - eventually the precipitate from electrolysis will clog and short the PEM stack.
Urine's major constituent is urea, which incorporates four hydrogen atoms per molecule - importantly, less tightly bonded than the hydrogen atoms in water molecules. Our generators use electrolysis to break the water molecule apart, with an inexpensive new nickel-based electrode to selectively and efficiently oxidize the urea. To break the molecule down, a voltage of 0.37V needs to be applied across the cell - much less than the 1.23V needed to split water.
In running an engine, using an alternator to supply the power to drive electrolysis - this makes a huge difference in how much gas can be produced per minute, which means a larger engine can be run under load.
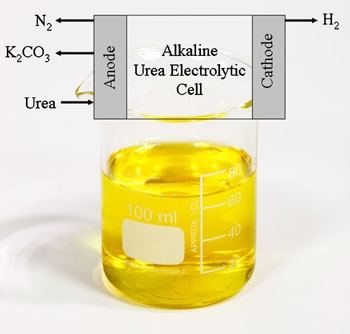
During the electrochemical process the urea gets adsorbed on to the nickel electrode surface, which passes the electrons needed to break up the molecule. . Pure hydrogen is evolved at the cathode, while nitrogen plus a trace of oxygen and hydrogen were collected at the anode. While carbon dioxide is generated during the reaction, none is found in the collected gasses as it reacts with the potassium hydroxide in the solution to form potassium carbonate.
Eventually the potassium carbonate settles as a part of a precipitate and is flushed out of a properly designed Hydrogen generator to be filtered away.
This was initially tested as process with 'synthetic' urine made of dissolved urea, - which is effectively what Diesel Exhaust Fluid (DEF) can be considered, but frankly the process works just as well with real human urine.
One of the reasons DEF is expensive is because processes that can remove urine from water are expensive and inefficient. One such method of isolating urea from urine is to boil down the urine to concentrate the solution, then add nitric acid. Urea nitrate is relatively insoluble and precipitates, then basically it is turned back into a solution with deionized water.
However Urea naturally hydrolyses into ammonia before generating gas phase ammonia emissions. These emissions lead to the formation of ammonium sulfate and nitrate particulates in the air, which cause a variety of health problems including chronic bronchitis, asthma attacks and premature death.
it is thus important to keep the Urine as sterile as possible and use it as immediately as possible after excretion. Urea gets converted very quickly into ammonia by bacteria, which could limit the usefulness of the technique, thus it is imperative to sterilize the Urine immediately - and keep it so in any storage tank as our system is deigned to do
The technology could be easily scaled-up to generate hydrogen while cleaning up the effluent from sewage plants. .This is an efficient way of producing hydrogen from urine - a feat that could not only fuel the cars of the future, but could also help clean up municipal wastewater.
Using hydrogen to power engines and vehicles has become an increasingly attractive transportation fuel, as the only emission produced is water - but a major stumbling block is the lack of a cheap, renewable source of the fuel and the dangers of carrying high pressure Hydrogen around in a bottle in a vehicle.. The answer, is using an electrolytic approach on demand to produce hydrogen from urine - the most abundant waste on Earth - at a fraction of the cost of producing hydrogen from water.
We sell parts and plans to build an ideal generator to do this also
| Attachment | Size |
|---|---|
| urine_electrolysis | 21.47 KB |